Blue Ice Fermented Cod Liver Oil Is Ita Fraud
Many people have been asking me to weigh in on the controversy that is erupted this week with the publication of Dr. Kaayla Daniel's report arguing that fermented cod liver oil (FCLO) produced by Green Pasture is rancid, low in fat-soluble vitamins, made from pollock rather than cod, and adulterated with trans fat-containing vegetable oil. While I would ordinarily want to thoroughly research all the unanswered questions in my mind before publishing something, the requests for my thoughts have been numerous enough that I would like to publish them as they currently are.
First, I would like to disclose potential contributions to an appearance of conflict of interest, and then briefly introduce how my thoughts had evolved over time on this matter in the years leading up to Dr. Daniel's report.
Potential Conflicts of Interest
Dave Wetzel has given me a large amount of free cod liver oil and skate liver oil over the years. If I remember correctly, years ago he paid me for permission to publish an article I had written about cod liver oil on his site. I had already written the article, I did not own the rights to it, and he could have legally published it on his site without asking permission, so technically that was a small monetary gift. Overall, I consider the value of these gifts as a proportion of my income to be negligible.
I have had a contractual relationship with the Weston A. Price Foundation (WAPF) for years, in which I receive compensation as an independent contractor for various forms of writing, speaking, and consulting. This constitutes a minor but significant proportion of my income. WAPF also paid for a semester of undergraduate courses I needed to apply for graduate programs in 2006 and funded my postdoctoral research at the University of Illinois. Years ago, I was a chapter leader for the Foundation, which is a volunteer position. I am not and never have been an employee of the Foundation. The Foundation endorses the FCLO, and Dr. Daniel's report has called this endorsement in to question. At no time did I ever play any direct or, to my knowledge, indirect role in the decision to endorse the product.
I do not sell Green Pasture products or any products of their competitors, nor do I earn commissions for the sale of these products. No one at WAPF, Green Pasture, or any of Green Pasture's competitors asked me to write this blog post and I am not receiving any compensation from anyone for writing it.
Although I have always had total creative control over my Mother Nature Obeyed (MNO) posts and have always written and published them without any prior review by anyone in the Foundation, I am writing this blog post here rather than over at MNO to emphasize the complete independence of this post from my contractual relationship to WAPF.
Background: My Thoughts on Cod Liver Oil
I started using cod liver oil after reading Nutrition and Physical Degeneration and Nourishing Traditions, probably somewhere around early 2002. This was one of many dietary changes that I made during this time, which were, together, associated with radical improvements in my health.
I have never seen cod liver oil as a necessity; instead, I have seen it as a convenience. Price used it in his practice not because the healthy non-modernized populations he studied used it, but because it was a convenient way to increase the fat-soluble vitamin content in the diets of people who needed it. It provides retinol, the physiologically essential form of vitamin A, which can also be obtained from most animal livers, and, in smaller amounts from other animal fats, particularly butter and egg yolks. It provides vitamin D, which can be obtained from sunlight, many fish, and in lesser amounts from terrestrial animal fats, particularly butter and egg yolks. It provides EPA and DHA, of which I am mostly interested in obtaining DHA, and this can also be obtained from fatty fish and, to a lesser extent, from terrestrial animal fats. In general, all these are more available from terrestrial animals raised on grass and in the sunshine than from terrestrial animals raised on grain and in confinement. It is easier to add cod liver oil to an imperfect diet than to perfect the diet, and for many people the most balanced approach to obtain all of these nutrients will be to consume a small amount of cod liver oil while also trying to hit the other dietary bases more often than not, allowing the cod liver oil to relieve the need for dietary perfection.
I do not recall what dose of cod liver oil I used early on, but it was never my only major source of retinol because I had, from the beginning of my venture into this way of eating, been eating copious amounts of buffalo liver. I read Nutrition and Physical Degeneration even before I read Nourishing Traditions, and Price writes very clearly therein that cod liver oil is most beneficial in low doses but toxic at high doses. Further, exposure to Mary Enig's writings made me skeptical of large amounts of polyunsaturated fatty acids (PUFAs). Later, exposure to Ray Peat's writings made me even more skeptical. Peat and Enig disagreed over the value of cod liver oil because Peat took a more extreme stance against PUFAs. I never fully agreed with Peat, but I did come to believe that it is desirable to get adequate physiologically essential PUFAs (which I see as arachidonic acid and DHA) and fat-soluble vitamins (in this case, A and D) without unnecessarily increasing the total PUFA content of the diet.
This led me to use smaller doses of cod liver oil while avoiding polyunsaturated plant oils and getting additional vitamin A, if I felt I needed it, by increasing my consumption of liver. Years later, for example, I had slacked on my vitamin A intake and found myself with a buildup of scaly material in my scalp; suspecting vitamin A deficiency, I used a combination of daily cod liver oil and liver until it went away. In recent years, I have settled into the practice of using small doses of cod liver oil in the colder part of the year when it is harder to obtain vitamin D from sunshine and relying more on liver for my vitamin A during the warmer part of the year, during which I would use cod liver oil primarily as occasional immune support when I felt I needed it.
Nevertheless, there was definitely a very common "more is better" approach to cod liver oil within the community, especially in the early years. Dr. Ron was one of many people using multiple tablespoons of cod liver oil per day. One of my friends had been a patient of his and for a long time took 2-3 tablespoons on his advice, and later became concerned that she developed a thyroid disorder because of it. Another friend used doses like this and she was convinced it gave her food intolerances. From what I can recall, both of these instances occurred prior to the introduction of fermented cod liver oil. It was this second friend, Laurel Crosby, now a researcher at Stanford, who introduced me to the research showing that arachidonic acid metabolites are necessary for immunological tolerance to foods and that high doses of EPA from cod liver oil or fish oil could interfere with this. I have since incorporated this into my public lectures on cod liver oil.
Eventually, Dave Wetzel of Green Pastures released his fermented cod liver oil. Initially, it was one more option on the market. Eventually, it came to dominate. From talking to people in the hallways of the WAPF conferences and reading through emails that people send me, I got the impression that some people saw it as an improvement while some people felt they did not tolerate it as well or did not receive as much benefit from it. I suspect in both cases it was sometimes due to actual health-related reactions to the oils while sometimes it was due to the perceived naturalness of the product or its flavor. I imagine that a great many people who jumped on the cod liver oil bandwagon after Green Pasture began selling the fermented product exclusively took it as their first cod liver oil and might not have personal experience to compare and contrast fermented and unfermented cod liver oils.
My first introduction to a serious criticism of the ideas behind the fermented cod liver oil was in 2012 when the Rosita extra virgin oils were being prepared for the market, and I was copied on private email exchanges with individuals associated with that company arguing that cod livers cannot "ferment" only "decompose," and at a much later time, email exchanges questioning the finding of vitamin D2 in the FCLO. These products became competitors to Green Pasture products around the same time that I began delivering a regular presentation on cod liver oil at the regional Weston Price conferences in 2013. As a result, questions from audience members about which is better began in earnest at that time. I have always refused to take a clear position on these questions for two reasons. First, I thought it was great that high-quality fermented and unfermented products were becoming equally available to consumers and I didn't want to get involved in disputes where the dividing line so clearly sliced directly and unambiguously between two competing companies. Second, I genuinely didn't buy arguments for the superiority of either oil.
I in no way present myself as an expert on the historical use of cod liver oil. However, I have gotten the impression that the cod liver oil that led to dramatic improvements in the prospects for tuberculosis in the nineteenth century was the "brown" oil while the cod liver oil that led to dramatic improvements in the immunity to measles, bedside fever, and the common cold in the twentieth century was the "light oil." When I was researching changes in US cod liver oil imports over the course of the nineteenth and twentieth centuries, I found this interesting snippet from the 1904 Report of the Commissioner of Fish and Fisheries:
Few subjects connected with materia medica have provoked so much discussion as the comparative merits of the light and the dark grades of cod-liver oil. Formerly, the brown oil was considered superior in efficiency to the paler sorts, and was generally favored for medicinal purposes. In recent years, however, chemists have claimed that analysis does not reveal any substance in the dark oil which would account for greater beneficial activity than the paler grades are supposed to possess. While many physicians yet recommend the brown oil, the drift of public opinion seems to favor the pale oil, and certainly it is more popular with the patients.
Here is a picture of my hard copy with handwritten notes of mine probably taken in late 2012 or early 2013:

I do not have sufficient expertise in the history to evaluate this statement, but if it is true, it suggests to me that the nineteenth century successes with cod liver oil were primarily with the brown oil and that the twentieth century successes were primarily with the light oil.
Further, it strikes me as particularly fascinating that they cite the absence of anything in the oil found by chemists to support the contention of physicians that the brown oil is better. While the observations of physicians were not based on randomized controlled trials, at least they involved clinical endpoints that people knew existed, were readily observable to the naked eye, and that people cared about. Clinical observations from the nineteenth century cannot be taken uncritically, but they should be taken seriously. That chemists couldn't find something in the nineteenth century should be dismissed without any consideration at all. Chemical analysis had yet to discover even the existence of vitamins, and it would be years before anyone would make a convincing case that anything beyond protein, carbohydrate, and minerals were essential in the diet. And yet it is almost certainly the vitamins in the cod liver oil, unknown to early chemists, that played the primary role in boosting immunity.
Based on this rudimentary understanding of the issue, I have held the impression that both oils are beneficial and that the reasons for one or the other being favored through history have never been strong ones. Consequently, I have always thought it best that consumers simply have access to both types of oil and decide for themselves which they would prefer to purchase.
With the publication of Dr. Daniel's report, this controversy has exploded, and, although I do not believe I have all the answers, I feel finally compelled to address the issue to the best of my ability. What follows is my preliminary response to her report. Whether I write more will depend on whether further information arises that compels me to modify or further develop my thinking on the issue.
The Semantics of "Fermentation"
The report begins with an eight-page discussion of the semantics of the word "fermentation," arguing that the oil cannot be fermented because fermentation necessarily involves carbohydrate. Dr. Daniel refers to the idea that cod livers can be fermented as "ludicrous on the face of it" according to the following reasoning:
The idea that cod livers could be fermented is ludicrous on the face of it. Lactic acid bacteria show a stunning capacity to degrade different carbohydrates and related compounds into the primary end product of lactic acid. But they have very limited capacity to synthesize the amino acids of protein and none at all to synthesize fats!
Fermentation requires carbohydrate, which is why it is most often associated with vegetables, grains, and dairy.
Compare this definition to the broader and more forgiving discussion in the microbiology textbook I have on hand (Tortora, et al., 2007, Microbiology: An Introduction, p. 137):
What is fermentation?
To many people, fermentation simply means the production of alcohol: grains and fruits are fermented to produce beer and wine. If a food soured, you may say it was "off" or fermented. Here are some definitions of fermentation. They range from informal, general usage to more scientific definitions. Fermentation is
1. Any spoilage of food by microorganisms (general use).
2. Any process that produces alcoholic beverages or acidic dairy products (general use).
3. Any large-scale microbial process occurring with or without air (common definition used in industry).
4. Any energy-releasing metabolic process that takes place only under anaerobic conditions (becoming more scientific).
5. Any metabolic process that releases energy from a sugar or other organic molecule, does not require oxygen or an electron transport system, and uses an organic molecule as the final electron acceptor (This is the definition we use in this book.)
Of these definitions, only #2 could imply an absolute requirement for carbohydrate, because glucose is the precursor to lactic acid and ethanol in most industrial uses. Lactic acid is most directly derived from pyruvate, which is the end product of glycolysis, a process that more or less splits a glucose molecule in half; amino acids and the glycerol component of fats can also be converted to pyruvate, so I doubt that sugar is the universal precursor for microbial lactic acid production, but it is the overwhelmingly usual precursor to lactic acid in all well characterized lacto-fermented foods.
In any case, definition #2 is a "general use" definition that isn't considered scientific by this textbook and is much more restrictive in nature than both the other general use definition and the industrial definition.
The textbook uses definition #5, which is a biochemical definition, and lists the following as substrates for fermentation: "sugars or other organic molecules, such as amino acids, organic acids, purines, and pyrimidines" (p. 134).
It seems to me that in the communities that value fermented foods as health foods we tend to use a definition similar to #1 but with the opposite connotation, implying the process produces a healthful product. Most of us would refer to sauerkraut as fermented cabbage, but in the biochemical sense (definition #5), the cabbage doesn't ferment, only its carbohydrate (and maybe some other minor substrates) does. It is the whole of the microbial metabolism responsible for the characteristics that differentiate sauerkraut from cabbage, and it is that (subjectively) desirable microbial metabolism that we usually have in mind when we colloquially use the word fermentation.
The biochemical definition (#5) is not limited to microbes or to food. When we engage in high-intensity exercise, our muscle cells ferment glucose to lactic acid. While a scrawny guy may be taunted with the question, "bro, do you even lift?" he would never be taunted with "bro, do you even ferment?" A technically accurate response to such a question might be, "Nah, bros can't ferment, just my glycogen does when my workout is all glycolytic." No one looks at the person breathing hard after a heavy exercise session and calls the person "fermented," but the purpose of breathing hard is to obtain enough oxygen to get rid of all the lactic acid that had been produced by muscular fermentation during the exercise.
Conversely, microbial metabolism is not limited to fermentation. Microbes possess cellular respiration, which fully breaks down carbohydrates, fats, and proteins for energy (Tortora, p. 137-8).
When we talk about fermentation in the context of microbial metabolism of food, the whole of microbial metabolism includes the use of lipids and proteins for energy, not just carbohydrate. When we talk about it in the biochemistry sense, it is not necessarily even related to food. I like the approach that my textbook uses, to recognize the diversity of uses of the word. It follows from that approach that we should use common sense when interpreting what someone means when they use the word.
According to Green Pasture, the phrase "fermented cod liver oil" technically means the oil of fermented livers. In a colloquial sense of having been metabolized by microorganisms, this seems accurate. I imagine that fermentation in the biochemical sense has occurred in the livers to one degree or another. I assume there is some glycogen in the liver that ferments in this sense. I do not know whether there is carbohydrate in the starter mix used for the fermentation. I do not know what organisms are in the starter mix and feel confident that no one knows the full range of microbes that participate. Even when well characterized and standardized starter cultures are used in a fermentation, other organisms invariably become involved. I do not know if the amino acids or the glycerol of its fatty acids are fermented in the biochemical sense. To me, this is not a scandal: the microbial processes involved simply are not well characterized.
While I think a discussion of the different definitions and best uses of the word "fermentation" is meritorious, I do not think using the word according to one of its general use or industrial use definitions can be considered fraudulent. I also do not see how the specific mode of energy metabolism used by the microbes that metabolize the livers necessarily has any implications for the healthfulness of the oil, which can be studied independently by looking at what it contains and its biological effects. So in the specific context of an introduction to a report accusing Green Pasture of fraud and alleging that the FCLO is categorically unhealthy, the discussion strikes me as a red herring.
Potential Practical Implications of the "Fermentation" Question
Dr. Daniel does discuss some practical health implications of whether the livers are fermented. She notes, for example, that if they were fermented in a way that models how the Inuit buried meat until it became "high," there would be a botulism risk. She had the oil analyzed for pathogens, however, and reported that none were detectable. She criticizes the oil for having a pH less acidic than that of a typical lacto-fermented food, and notes that Dave Wetzel has reported even an even less acidic pH, and she concludes from this that the oil does not benefit from acidic preservation.
Technically, an oil does not have a pH: pH is a measure of the acidity of an aqueous solution. Regardless, this is a grossly oversimplified view of food preservation. Lactic acid fermentation preserves the "food" in the sense that it prevents spoilage by other undesirable microorganisms, but it does not preserve all of its components. It breaks some things down, synthesizes other things: changes the food. Some nutrients are best preserved by acid, such as vitamin C or thiols like glutathione. Some nutrients are best preserved by avoiding exposure to light, or to certain wavelengths of light, such as riboflavin and vitamins A and K. In an oil, we are primarily concerned with oxidative degradation of the polyunsaturated fatty acids (PUFAs). Coconut oil is stable over years because it is very low in PUFAs; this is true despite little antioxidant protection, especially in the refined oil, and despite no effort to preserve it with acidity. In a marine oil rich in highly unsaturated PUFAs, stability is primarily conferred by antioxidants. Liver is rich in all sorts of compounds that could act as antioxidants, such as thiols (protein thiols, thioredoxin, glutathione, etc), lipoic acid, and others, probably with a lot of variation in fat solubility and the ability to migrate into an oil extract. Microbial metabolism of the livers adds a whole layer of who-knows-whats-in-it. I imagine it would be time-consuming, arduous, and require a lot of intellectual creativity to fully characterize the compounds within the oil and determine what, if anything, is acting as an antioxidant.
The Semantics of "Rancidity"
While I consider the semantic discussion of "fermentation" in the beginning of the report to have little practical value, I wish more consideration had been given to the semantics of the word "rancidity." Throughout Dr. Daniel's report, the term "rancidity" is dealt with as if it had a singular meaning, and I believe the failure to distinguish between the different types of rancidity led to a flawed conclusion about whether the fats in the oil have been damaged. In this section, I will address the distinction itself. In the next section, I will address whether the FCLO is rancid.
In his 1954 47-page review, "Autoxidation of Fats and Related Substances," Ralph Holman, one of the most important giants in the history of lipid science, noted several distinct uses of the word:
Chemical changes of several types contribute to what is known by the generic term rancidity. In its broadest meaning, rancidity denotes a deterioration of flavour and odour of fat or the fatty portions of foods. Such deterioration can be due to hydrolysis, oxidation, or to microbial action. The term rancidity is used in the dairy field to indicate hydrolytic deterioration; in other fields it denotes microbial deterioration, and to the fat chemist it means autoxidation.
This more recent (2001) book also makes the distinction (p. 39). It defines "oxidative rancidity" (autoxidation or the related photooxidation) as the reaction between oxygen and the double bonds of unsaturated fatty acids. It continues:
Hydrolytic rancidity results from lipolysis of triacylglycerols in which the released fatty acids give a distinct taste to the oil. This is usually reserved for the lauric fats or butter to which the shorter chain fatty acids can give a soapy taste. The third form of rancidity is called ketonic rancidity and results from microbiological attack on lauric fats, which first liberates short-chain free fatty acids and then subjects them to a beta-oxidation, yielding methyl ketones and aliphatic alcohols. It is closely related to hydrolytic rancidity.
From a mechanistic perspective, these are three different and independent processes. From a food industry perspective, they are three independent ways to achieve the same undesirable result: off-flavors.
Perusing food industry-oriented textbooks suggests that the overwhelming if not exclusive concern about rancidity is its effect on flavor. For example, this book refers to lipid peroxidation products as "off-flavor volatiles." The quote above about hydrolytic and microbial rancidity is explicitly concerned with flavor. The same book later refers to tests for lipid peroxidation such as peroxide value, anisidine value, and TBARS as follows (p. 51-2):
The thinking here is that as double bonds oxidize they form peroxides (primary oxidation products), which, though odorless and tasteless, are precursors to a wide range of secondary oxidation products. . . . Sadly, there are few, if any, convincing studies that show good correlation between peroxide and or anisidine and taste panel sensory scores. . . . For triacylglycerols rich in LCPUFA, these methods are equally inappropriate. Sensory evaluation of the oil or food product containing the oil is the only reliable method of deciding whether the oil is fit for use, but this is no easy task and recruiting, training, and maintaining the panel can be difficult. . . . Factors associated with or even responsible for the fishy taste and smell of a marine oil are not well defined. There are many kinds of fish oil and many different ideas of what constitutes rancid fish oil. The strictest judges are the professional taste panels of the food industry, who can fail an oil that less experienced people might think has only just began to go rancid or that is perfectly suitable for food use.
When Holman wrote that the "fat chemist" is concerned only with oxidative rancidity, this is because his field was studying the biological health-related effects of the process. He identified three concerns: loss of antioxidants, loss of essential fatty acids, and the creation of toxic byproducts of damaged fatty acids.
This is not to say that the processes have no relationship to one another at all. Obviously if they are all happening simultaneously they will all get worse over time. Free fatty acids are, all things being equal, more vulnerable to peroxidation than fatty acids joined in ester bonds (as in triglycerides, for example). But hydrolysis of triglycerides to free fatty acids is not a requirement for peroxidation to occur, and peroxidized lipids will fragment into secondary oxidation products, not convert to free fatty acids. In tissues with enzymatic systems, such as muscle tissue, peroxidation can induce hydrolysis of free fatty acids from phospholipids in cellular membranes, but as far as I know this should not be relevant to a food oil.
Without ignoring some potential interrelationships, it is important that we recognize these as distinct processes when we consider the question of whether the FCLO is rancid.
Is the FCLO Rancid?
In asking the question of whether the fermented cod liver oil is rancid, my concern is specifically whether it demonstrates a history of lipid peroxidation. With a few minor exceptions discussed further below, Dr. Daniel found that the FCLO had acceptably low levels of lipid peroxidation products as measured by the peroxide, anisidine, Totox, and TBARS tests. However, the FCLO had high free fatty acids and a high acid value, which reflects the high free fatty acid content. This data is consistent with what Green Pasture has claimed about its product. However, Green Pasture has taken the absence of lipid peroxidation products to indicate the absence of rancidity. Dr. Daniel argues that the peroxide, anisidine, TOTOX, and TBARS tests are only useful for oils in early stages of peroxidation and takes the high free fatty acids as demonstrating a past history of peroxidation. In other words, the oil has been so extensively peroxidized that all of the peroxidation products have gone and the free fatty acids have shot through the roof.
Dr. Daniel summarized her argument for David Gumpert very similarly, as quoted here.
When I first read this, I was concerned. I had always found the claim that the FCLO was immune to rancidity to be difficult to believe, and it had crossed my mind in the past that the oil might not peroxidize at room temperature because there is nothing left to peroxidize. I never had the time to think deeply about it or look into it any further, so this remained nothing more than a passing thought and I kept taking the FCLO through the years.
But one thing just didn't add up, and caused my skepticism to grow. If we take at face value the claim that the secondary peroxidation products eventually degrade and become undetectable, we have to assume that the oil is so extensively peroxidized that these products are no longer being generated in detectable amounts. As antioxidants are depleted (and as free fatty acids increase, if hydrolysis is also progressing simultaneously), the fatty acids become even more vulnerable to peroxidation, so even if the peroxidation products were unstable, they should increase over time rather than decrease as the rate of peroxidation accelerates, until the peroxidation is so thorough that there are few PUFAs left to peroxidize.
I discussed this with Chris Kresser, and we came to the conclusion that the ~1 g/tsp of EPA and DHA seems relatively normal for a cod liver oil and doesn't seem indicative of such a scenario.
A few weeks ago, a specialist in lipid peroxidation had reached out to me to discuss EPA and DHA supplementation. I emailed him about this and sent him a copy of Dr. Daniel's report. He told me that although it is true the peroxide value will decline with time as the peroxides fragment into secondary peroxidation products, the indicators of those secondary products (anisidine, Totox, and TBARS) should remain high even in a very extensively peroxidized oil. He also agreed that the level of EPA and DHA was inconsistent with extensive peroxidation, and told me that free fatty acids are not an indicator of past peroxidation. He declined to be publicly acknowledged, however, so he remains an anonymous source.
I tried (within the limitations of the time I had) to find actual data on what happens to these values in an extensively peroxidized oil and was unable to find any. My anonymous source was confident from autoxidation experiments performed within his own laboratory that the assessment above is correct, but was unable within the limited timeframe and given his other responsibilities to point me toward published data demonstrating this.
I went back to Dr. Daniel's report to see if I could find references that contain this data. I first searched the two references given for the following statement: "At this point, anisidine values begin to climb, flavors and odors become objectionable, and consumers may experience burping. Next, anisidine levels, in turn, will decrease." The first reference describes anisidine values rising, but not falling. It shows this figure, in fact, that depicts the anisidine value remaining high over an extended period:
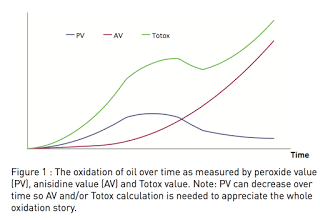
The figure does not show actual data. Instead, it graphically depicts the importance of measuring the anisidine and Totox values. As free radicals and oxygen initially attack PUFAs, they generate peroxides, and the peroxide value increases. As the peroxides fragment into secondary products detectable with the anisidine test, the anisidine value increases. When the rate of peroxide fragmentation finally exceeds the rate of peroxide formation, the peroxide value falls while the anisidine value continues to climb. The Totox is a calculation that combines the results of both tests, so it climbs throughout the process.
The caption of the figure says that the Totox calculation "is needed to appreciate the whole oxidation story." The same references goes on to describe the acid value as indicating the cleavage of free fatty acids, which "reveals enzymatic activity due to microorganisms in the raw material."
This reference does not support and seems to contradict Dr. Daniel's contention that the anisidine and Totox decrease with extended peroxidation and that free fatty acids indicate past peroxidation. It seems, moreover, to support the concept that the free fatty acids in the FCLO reflect microbial digestion of the oil.
The second reference is a book. The cited pages (220-222) are not available in a Google Book or Amazon preview, and the book is expensive. It seemed doubtful that these three pages had the smoking gun of actual data showing the decline of the anisidine value with extensive peroxidation, but I did not want to miss that possibility. So I emailed Dr. Daniel about this and asked if she could clarify how she came to this conclusion, and, if the book was critical to the conclusion, that she send me copies of the relevant pages. She responded that it was difficult to find data on such extensively peroxidized oils in the literature or in books, so her conclusions about the oxidation products eventually falling to normal levels in such oils were derived from personal conversations with experts in the field.
It is worth noting that the aforementioned tests (their limitations discussed in more detail below) are extremely crude, rough-around-the-edges tests that are used so extensively because they are inexpensive and can be run with widely available equipment. WAPF sent samples to Dr. Martin Grootveld of the Leicester School of Pharmacy, who performed more sophisticated NMR-based techniques to analyze the oil for a range of peroxides and aldehydes, finding none detectable. The results are available here.
Grootveld's group published a paper where they used this method to detect high levels of toxic aldehydes in restaurant fryer oil and in commercially available PUFA-rich oils that they experimentally subjected to heat. They found only low levels when they subjected commercially available olive oil, coconut oil, lard, and beef fat to the same heating process. These aldehydes are secondary products of lipid peroxidation and they correspond to what would be picked up by the anisidine value test. The difference is that the anisidine value test is comparatively cheap, easy, and non-specific, whereas the NMR test is comparatively expensive, difficult, and very specific. The Grootveld group noted in their conclusion that primary lipid peroxidation products are not absorbed in the diet but secondary products are, and it would thus seem that high concentrations of the secondary products are what we should consider to be of toxicological interest.
Notably, the Grootveld group stated within that paper that they found primary oxidation products (corresponding roughly to the peroxide value test) in linoleic acid that they kept in the dark exposed to atmospheric oxygen for about three months. This suggests that under such conditions it takes much longer than three months for the peroxides to decline to normal levels. If it is true that the secondary peroxidation products eventually do the same, it would have to take even longer.
While I am willing to consider arguments that the secondary peroxidation products would disappear in an extensively peroxidized oil, I am unconvinced of this claim for the time being. While I do not think the presence of relatively normal EPA and DHA levels indicates in and of itself that no peroxidation has occurred, I think it indicates clearly that the peroxidation has not been extensive enough to wipe them out as material for continuing peroxidation. If the oil is undergoing peroxidation and there is plenty of material to continue to peroxidize, I do not see how markers of peroxidation could drop to acceptably low levels. I do not see any convincing evidence that free fatty acids are clear signs of past peroxidation. Altogether, then, I do not think the oil demonstrates a history of lipid peroxidation, and in the sense of the "fat chemist," I do not believe it is rancid.
While this is my conclusion about Dr. Daniel's central argument, I will discuss two caveats below. First, I would like to address whether the free fatty acids, agreeable by all parties to be present, are harmful to consume.
Are Dietary Free Fatty Acids Toxic?
Dr. Daniel states that "Free Fatty Acids are so cytotoxic to cell membranes that the body makes it a priority to build them back into the safe triglyceride form as quickly as possible." This statement is part of a brief discussion suggesting that the FCLO is harmful because of the free fatty acids themselves. The potential toxicity of free fatty acids is true, but is pulled grossly out of context.
When we digest food, we hydrolyze fats to free fatty acids and monoglycerides before absorbing them into the intestinal cell, where they are repacked into triglycerides. Some authors argue that long-chain omega-3 fatty acids are more bioavailable when supplemented as free fatty acids. This is controversial, but it is notable that there are scientists even conducting studies of free fatty acid supplementation, which would not be the case if it were obviously harmful to consume free fatty acids.
Free fatty acids elevate within our bodies in both good and bad contexts.
When we burn fat for energy, whether derived from our diet or our adipose tissue, we hydrolyze fats into free fatty acids so we can break them down during beta-oxidation. This increases in the context of fasting between meals, exercise, or consumption of a diet richer in fat or lower in carbohydrate. These processes are normal and healthy.
In the stress response, our body senses the need to release large amounts of stored fat, so free fatty acid levels elevate. Insulin prevents the release of free fatty acids, so in insulin resistance free fatty acids also elevate. These free fatty acids can compete with glucose for energy metabolism and thereby contribute to elevated glucose. They can also inhibit the biological activity of thyroid hormone. If cells are overwhelmed with more free fatty acids than can be efficiently burned for energy, they can also have toxic effects and increase the vulnerability to oxidative damage. But this is not an intrinsic toxicity of free fatty acids; it is the harm they cause during metabolic derangement.
Clearly, eating free fatty acids is quite a different phenomenon than free fatty acids rising endogenously. But it is not that different a phenomenon from eating triglycerides, which we break down into free fatty acids before absorbing them anyway. The main difference is that when we eat free fatty acids they have already undergone that step of digestion.
Rancidity Caveat #1: Dave Wetzel on the Peroxide History of his Oil
Dr. Daniel cites a video in which Dave Wetzel discusses the time-dependent peroxide value history of his oil, wherein he says that it rises to a high level and then returns to normal before he bottles the oil. In response to the Daniel report, Wetzel released data in pdf form on the same topic. He had told me about this phenomenon years ago, and, as noted above, it had crossed my mind that this was harmful. In reviewing it now, the phenomenon does sound eerily similar to the natural course of peroxide formation where it increases until the peroxides degrade into secondary products that are toxic. Yet Wetzel's data show that the anisidine value, an indicator of secondary peroxidation products, never rises to unacceptable levels over the same time course.
A few possibilities would be consistent with this: 1) the peroxides did not rise high enough to generate unacceptable levels of secondary peroxidation products, 2) the peroxides formed and then were converted to something other than secondary peroxidation products, or, discussed in more detail below, 3) the peroxide value test was picking up something besides peroxides.
Dr. Daniel's data show no evidence of unacceptable anisidine values. She infers from Wetzel's video and from the high free fatty acids that the anisidine value had been high and has since declined. But Wetzel's data shows no history of unacceptably high anisidine values, and if we take his peroxide value data seriously we should also take his anisidine value data seriously.
The peroxide value test has a major limitation: it isn't a specific test for peroxides. In fact, the peroxide value test is the activator X test of Weston Price. This is such an important point and so easy to lose sight of that I feel the need to emphasize this by repeating it three times:
- The peroxide value test is the activator X test.
- The peroxide value test is the activator X test.
- The peroxide value test is the activator X test.
In my 2007 article On the Trail of the Elusive X-Factor: A Sixty-Two Year Old Mystery Finally Solved, I described this in detail in the sidebar, "The Activator X Test." It not only detects peroxides, but anything capable of oxidizing iodide to iodine, including quinones such as coenzyme Q10, and, almost certainly, vitamin K. The principle of the test as currently used is the same as in Price's day, and its limitations remain the same.
Green Pasture reports significant quinone content in the FCLO. I am not familiar with the quinone test so I am not in a position to describe its strengths and limitations. But, I feel confident that if the quinone data is accurate, then the peroxide value test is almost certainly registering those quinones as "peroxides."
Does the quinone content, or the content of anything else capable of oxidizing iodide to iodine besides lipid peroxides, vary over the course of the processing of the oil due to microbial activity? I don't know, but nothing seems implausible about it.
I find Wetzel's description of the peroxide value test concerning enough that I want to see these questions elucidated, but not damning enough to convince me that the oil is extensively peroxidized in the face of no evidence of secondary peroxidation products.
Rancidity Caveat #2: Two Aberrant TBA/MDA Tests
Although the Daniel report primarily shows an absence of secondary lipid peroxidation products, there are two outliers cited: one out of three TBARS tests done for the report showed a high value, and a conference abstract published by Standard Process Laboratory found 10 times more "MDA" in a fermented cod liver oil (presumably the Green Pasture product, though not specifically named) than in other cod liver oils.
Before discussing these results, it is necessary first to give some background about the TBARS test. Although this test is often referred to as a test for malondialdehyde (MDA), one of the secondary lipid peroxidation products, this is erroneous. MDA is only one of many compounds that are picked up by the test. TBARS stands for TBA-reactive substances. TBA stands for 2-thiobarbituric acid. In the assay, you mix TBA with the sample and heat it. The more substances in the sample that react with TBA, the more intense of a pink color is formed. Finally, you stick the sample in a machine called a spectrophotometer, which measures the intensity at which your sample absorbs light at a specific wavelength.
The very name of this test — TBA-reactive substances — is an admission that it is not a measurement of MDA, or of any specific chemical.
This test is widely criticized for its lack of specificity and for the possibility that colored components of a sample could cause interference. The fact that you have to heat the sample to cause the "substances" to react with TBA is also concerning, because heating oils can cause the formation of MDA and other TBA-reactive substances.
I have some personal experience measuring MDA. In my doctoral lab, we would measure MDA using high-performance liquid chromatography (HPLC). It is a similar procedure, except that we would inject the sample into a HPLC, which would then separate all of the individual components. This allowed us to separate MDA from all the other TBA-reactive substances and quantify the MDA specifically.
We would always add butylated hydroxytoluene (BHT), an antioxidant, to the sample while heating it to prevent the formation of MDA during the procedure. Protocols for the TBARS test do not call for this, which makes me think that MDA and other TBA-reactive substances are probably created during the typical procedure. Furthermore, carrying out the procedure on 100% analytical grade ethanol would make the ethanol turn pink and generate a considerable amount of "MDA." I doubt that 100% analytical grade ethanol has MDA in it. In all likelihood this wasn't actually MDA, so we would always run an ethanol blank to appropriately adjust for the noise.
I find the TBARS test difficult to take seriously. It may be a rough but decent first indicator of peroxidation when comparing the same type of oil over time or between batches, but it should not be used to infer peroxidation without confirmation from other tests. Certainly, it should never be referred to as a test for MDA, because it is not specific to MDA. The probability of generating a false positive seems high, and this is especially true without proper care to prevent artifactual formation of TBARS. Finally, comparing different oils with different pigmentation strikes me as questionable.
The Standard Process abstract says MDA was measured with a "calorimetric assay." That they weren't separating the MDA chromatographically indicates to me that they were using the TBARS test and calling it "MDA." Thus, "MDA" should not be used without quotation marks when referring to this study. Dr. Daniel states in the associated footnote of her report that "The study was only published as an abstract and Standard Process declined to share the complete study or specific data." I therefore did not try reaching out to them to obtain the same information. In this case, they were comparing a fermented cod liver oil (presumably the Green Pasture product, but this is not stated) to other cod liver oils. But certainly these cod liver oils are different colors, so to run a calorimetric (color-based) assay on them without chromatographically separating the components strikes me as highly questionable.
One of the three labs that Dr. Daniel had analyze the FCLO found high TBARS, while the other two found very low TBARS. She cites three TBARS values from the Green Pasture web site that were all low. WAPF lists eight TBARS results that were all very low.
If the same lab had found most batches to have low TBARS but certain batches to have high TBARS, I would be inclined to think that the aberration was a result of interbatch variation, that some batches may have been spoiled, and that more quality control was needed. If a number of laboratories find low TBARS and one finds high TBARS, I'm inclined to think the aberration is an analytical error. This case seems closer to the latter.
Altogether, it seems to me the balance of the evidence favors the conclusion that the FCLO has not undergone a significant history of lipid peroxidation and does not contain toxic lipid peroxidation byproducts.
Biogenic Amines
After discussing rancidity, Dr. Daniel moves on to the topic of biogenic amines. I agree with most of the material in this section, except the implication that the presence of amines in the FCLO means it is a poor quality product.
Dr. Daniel cites Green Pasture's claim that the tyramine content ranges from 5 to 50 ppm. Two batches manufactured in late 2013 or early 2014 and submitted by Dr. Daniel to labs #2 and #4 showed either no detectable amines (lab #4) or low levels of amines (lab #2). A batch manufactured in 2012 and submitted in August, 2013, to lab #7, found very high levels of amines, including putrescine and cadaverine, often associated with putrefaction or rotting.
I do not have any expertise in testing amines and cannot comment on the strengths and limitations of the tests involved. It is unclear whether the disparity in the results reflects differences in laboratory methods, differences in laboratory competence, or difference in amine content between batches.
According to data assembled by WAPF, some cheeses have considerably higher levels of amines, even putrescine and cadaverine, than what lab #7 in the Daniel report reported for FCLO.
Not every fermented food is good for every person, and some people don't tolerate fermented foods well at all. I think this is largely mediated by the biogenic amine content. I suspect that some negative reactions some people experience with FCLO are due to the amine content, and I find this far more plausible than that people are being harmed by toxic byproducts of lipid peroxidation in the oil.
Fat-Soluble Vitamins
The next section of the Daniel report argues that the fat-soluble vitamin content of the FCLO is low. A appears significant, but lower than claimed by Green Pasture; E and K are very low, but this is not very surprising for a cod liver oil; D is surprisingly low.
I am not going to comment extensively here, because I have an article on the nature of vitamin D in cod liver oil coming out in the next Wise Traditions. This has been in store for a while, so it is not a direct response to Dr. Daniel's report, but it deals with the same topic, and I don't want to spill the beans before it's out nor be redundant. I also discussed this topic with Chris Kresser, and he wrote about our conversation already. I will therefore address this topic briefly.
The vitamin D activity in cod liver oil is likely from a variety of vitamin D metabolites. Dr. Daniel collected laboratory data using very high quality methods of analytical chemistry, and they are probably very accurate about the vitamin D2 and D3 contents of the samples. This does not mean that they accurately convey the biological vitamin D activity. Wetzel submitted his FCLO for a test of its ability to prevent rickets in rats, and this test found high levels of D activity.
Dr. Daniel dismissed this on David Gumpert's blog according to the following reasoning:
As for Vitamin D absorption in rats, Daniel says, "Gall bladders are
critical for the assimilation of fats and fat-soluble vitamins in
humans. Rats don't have gall bladders so the studies Wetzel cites have
questionable applicability to humans.
It's true that the biological vitamin D activity of the FCLO could be different between rats and humans, but not having a gall bladder does not allow a rat to absorb something that does not exist. So if high quality analytical methods show that there is not enough "vitamin D" to cause these effects in rats, there must be other compounds with vitamin D activity that are doing the job.
Interestingly, the Standard Process data linked to above in the discussion of TBARS and MDA found that only their fermented cod liver oil sample and crude cod liver oil samples contained vitamin A2, or dehydroretinol. While I think the vitamin A story is less complicated than the vitamin D story, the existence of vitamin A in this unusual form within marine life could perhaps cause similar errors in underestimating the vitamin A potency of an oil.
Dr. Daniel criticizes Wetzel's speculation that the quinones in his FCLO could possess vitamin K activity and supports this criticism with data showing that it is low in vitamin K. I agree that, at this time, it cannot be claimed that these quinones do possess vitamin K activity, but showing that they are not vitamin K does not disprove this. Analytical chemistry can explain biological effects, but it cannot confirm or refute them. Biological effects are shown with tests of biological effects. At present, the idea remains an interesting speculation.
Pollock Not Cod
Dr. Daniel argues that the FCLO is made from Alaskan pollock rather than cod. This is based on a higher than expected ratio of EPA to DHA more reflective of pollock than cod, and DNA analysis showing that the livers used for his Cattle Lick product are Alaskan pollock. Of these, the DNA analysis seems most reliable.
Assuming that some or all of the FCLO is made from Alaskan pollock as this suggests, the ethics of the labeling is well beyond my area of expertise. I know from reading old papers that pollock was legally considered cod liver oil in the 1930s (as discussed here, for example), but I don't know what the current laws require or what the current industry standard is.
Certainly, this warrants an explanation from Green Pasture. I will await hearing from Green Pasture before coming to an opinion about the legal and ethical issues surrounding this question.
I do think it is self-apparent that consumers benefit from greater transparency about the sources of a product. If Green Pasture were to determine that there is no ethical or legal responsibility to distinguish between species in the cod liver, I would hope they would begin clarifying this in their labeling anyway.
Trans Fats
Dr. Daniel also reports 3.22 percent trans fat in the oil, the majority of which is 18:3 trans, presumably derived from 18:3 cis (alpha-linolenic acid). Since pollock liver oil does not contain enough 18:3 cis to account for this 18:3 trans, she argues, it must be added.
She concludes that this must have come from a thermally damaged vegetable oil that Wetzel is adding to the FCLO. She then considers the possibility that Wetzel is taking the cost-cutting step of buying bottled pollock liver oil that, unbeknownst to him, is diluted with thermally damaged vegetable oil, then fermenting that oil. In such a case, she argues, he would be fermenting livers for the Cattle Lick product purely for the sake of duping visitors into believing that the FCLO is made from fermented livers.
The first possibility strikes me as reasonable speculation; the second as wild speculation.
If the test results are accurate, then the fatty acid must come from one of the following: 1) the diet of the fish, 2) endogenous synthesis by the fish, 3) microbial production, 4) adulteration.
I find 1 and 2 less plausible than 3 and 4 simply because if 1 or 2 were true, the fatty acid should be regularly found in these fish at similar concentrations, though I would not consider them impossible. Given how little we know about the microbial process involved, and the rapidity with which our understanding of microbes is constantly expanding, 3 strikes me as quite plausible. 4 is certainly simpler, because it is well established from common experience that producers may adulterate their products. Fraud is a major accusation, however, so while evidence of its possibility should by no means be swept under the rug, it makes sense to me that Green Pasture deserves the chance to respond and the benefit of the doubt until dialog or debate can lead to a firm conclusion.
I think it is worth noting that the lab test does not seem to distinguish whether the 18:3 trans is all-trans, or is conjugated with alternating cis and trans double bonds. I think further elucidating the nature of the fatty acid may help shed light on where it comes from.
Is the Butter Oil Rancid?
Data from the Daniel report show the butter oil has high free fatty acids and a high peroxide value. The free fatty acids in that sample indicate hydrolytic rancidity, not a toxicological concern, but probably able to explain the off flavors some people reported. The peroxide value could indicate peroxides, but since the peroxide value test is the activator X test, so it could also indicate vitamin K and other quinones.
Vitamin K: Still Activator X?
One of the last conclusions in the report is that vitamin K2 is probably not activator X after all.
I argued that it was in my 2007 article, On the Trail of the Elusive X Factor: A 62-Year-Old Mystery Finally Solved.
Here, Dr. Daniel argues that since butter oil does not seem to contain any more vitamin K2 than butter, activator X is probably not vitamin K2:
This strongly suggests that vitamin K2 might not be Dr. Price's elusive "X Factor." Dr. Price was specific that it was butter oil — and not just butter — that had healing power and a potent synergizing effect on cod-liver oil. Clearly, it's time to test a variety of butters, butter oils and ghees not only for vitamin K but for other potentizing factors.
Until then it looks like vitamin K2 is not Dr. Price's mysterious "X Factor" after all.
Price used large amounts of butter in his protocol and small amounts of butter oil. The purpose of the butter oil was to concentrate the activator X, not to uniquely supply it. He quantified activator X with the peroxide value test, which is not a specific test for any chemical compound, but picks up a variety of compounds. Presumably his conclusions about the value of producing the butter oil were based both on the observation that the "activator X" test value was higher and that it seemed more potent in his animal experiments and clinical experience. Given the lack of specificity of the test and the difficulties of discerning cause and effect of specific components in the context of complex interventions, I think we need to take all of Price's clinical observations seriously, but not uncritically, and I don't think it makes sense to let the identity of activator X hang on any one statement that Price made.
That said, the two most relevant questions here are 1) is the Green Pasture process for making the butter oil the same as Price's? and 2) is it concentrating the vitamins from the cream used to produce it? The correct way to determine the latter is to compare the vitamin value in the cream to that in the butter oil, or to compare the process of making butter from that cream to that of making butter oil from that cream.
My argument that vitamin K2 is activator X was based on a wide range of evidence including the biological and chemical behavior of the vitamin in addition to its distribution in foods. I welcome thoughtful critiques and counter-arguments, but something larger than a comparison between a few different butter and butter oil products is needed, in my view, to dismiss my argument wholesale in this way.
Conclusion
In an ideally functioning market, competition between producers will lead to competition for greater transparency and greater quality. When consumers are critical and some people act as watchdogs, this helps the market function in that way.
I think competition among cod liver oil suppliers in the ancestral health community is a good thing, and I think critical analysis of these products is a good thing. Toward that end, I appreciate Dr. Daniel for offering a critical analysis of the Green Pasture products.
At the same time, I find the antagonistic tone of the report to be unfortunate, and many of the accusations reach beyond what the evidence should allow for. If this report stands on its own, I do not think Green Pasture gets a fair hearing. However, if this report initiates a reasonable dialog about these topics and leads to a greater supply of information through more rigorous and sophisticated testing and a competition between companies for greater openness and transparency, I think everyone will benefit.
Toward that end, I hope my critique of the critique contributes well to that type of dialog.
Source: https://chrismasterjohnphd.com/blog/2015/08/29/weighing-in-on-fermented-cod-liver-oi/
0 Response to "Blue Ice Fermented Cod Liver Oil Is Ita Fraud"
Post a Comment